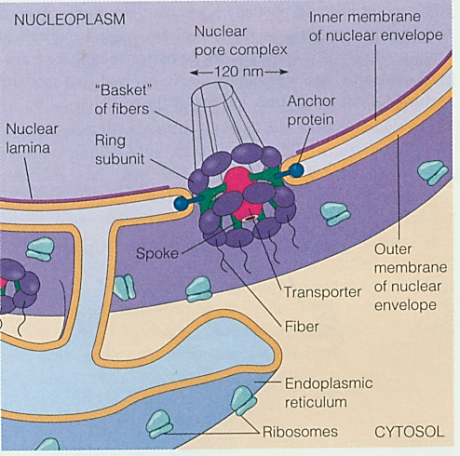
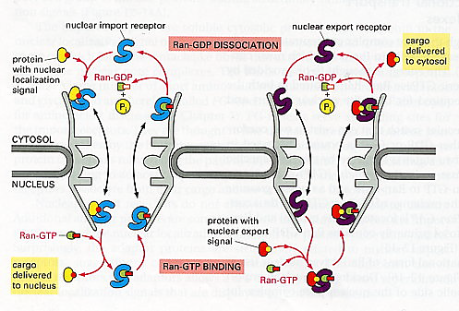
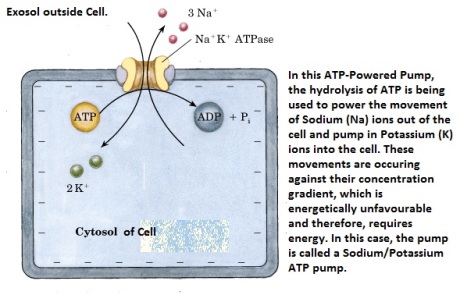
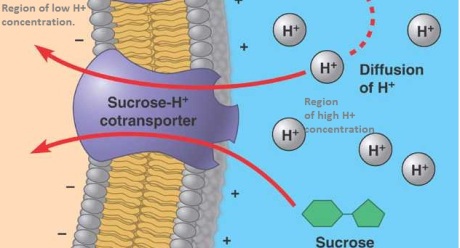
Extracellular matrix (ECM) is the material found around cells and biochemically partly made by the cell. It is composed of complex mixtures of proteins, proteoglycans, and, in the case of bone tissue, contains mineral deposits as well.
- Protein; almost all of the proteins found in the ECM are glycoproteins which include any of a group of complex compounds containing sugar units or polysaccharides combined with amino acid units or polypeptides. Some examples are collagen, laminin and fibronectin. Elastin is not a glycoprotein but is simply a protein and provides flexibility to skin arteries and bones.
- Proteoglycan; are partially similar to glycoproteins but are made up mostly of carbohydrate consisting of various polysaccharide side chains linked to a protein, and resemble polysaccharides rather than proteins in their properties. They usually have groups of carbohydrate chains attached to a protein backbone.

Typical Proteoglycan. Click on image for credit.
- Mineral deposits; found in bones such as calcium and phosphorous.
Most normal cells cannot survive unless they are anchored to the extracellular matrix.
Cells attach to the ECM by means of integrin (integral membrane proteins) which are transmembrane glycoproteins that bind to the ECM proteins collagen, laminin and fibronectin (can exert a physical force on the cell). Internally, the integrin binds to actin filaments of the cytoskeleton. This gives them a key structural role in cells (more on this in chapter 6).
Cells don’t simply squeeze next to each other in a tissue because if they did, they might temporarily hold together but wouldn’t be stable enough to compose tissue as they’d fall out due to lack of adhesion. They are connected to each other via membrane-associated structures (structures situated on the plasma membrane), usually transmembrane proteins. These membrane-associated structures help in cohesion and communication. Where cells stick tightly to each other (adhesion), this is done with the help of Cadherins, a family of transmembrane glycoproteins. Not only does it stick cells together, it also helps seal junctions between cells to prevent the flow of materials through the intercellular space.
There are many types of intercellular junctions situated at various spots on the cell:
1. Tight Junctions or Zonulae Occludens (singular Zonula) situated apically. Zonula is Latin for encircling and Occludensto enclose- so referring to the fact that the junction forms a band completely around the cell fusing cells together and closing-off intercellular space. The number of fusion sites (viewed as ridges and grooves like a suture) often correlate to the leakiness of the cell tissue. The less fusion sites, the more permeable to water and solutes and vice versa. Ex; Cells of the proximal renal tubule of the kidney where filtration often takes place have few fusion sites to allow for leakability, whereas, cells of the urinary bladder (that must hold urine and not allow it to leak out) contains numerous fusion sites or tight junctions to form a seal to prevent the flow of materials between epithelial cells.

Intercellular Junctions. . The cell in the middle was emptied of it’s contents to show the inner surface of it’s membranes.
2. Intermediate Junction or Zonulae Adherens situated at close proximity to the apex but somewhat below the tight junctions, also encircles the cell and provides adhesion of neighboring cells to each other due to the insertion of numerous actin filaments from the cytoplasm of the cell into the junction forming a network. But, unlike tight junctions, they are relatively permeable as there are no fusion sites that completely seal off the paracellular space. The two Zonulas form a continuous ribbon around the cell apex.
3. Gap junction situated almost anywhere along the lateral surface of the epithelial cell is simply a gap between the neighboring cell membranes that is about 2nm. Contains protein unit called connexin (a hexamer: polymer made of six monomers) with a hydrophilic pore (1.5nm diameter) that acts as a hydrophilic ion channel between two cells. Thus, an individual unit of the gap junction is called a connexon (many seen in image).

Connexons. Gap Junction.
4. Desmosome or Macula Adherens can be situated anywhere on the lateral surface (or sides) as well. Macula means a patch so it doesn’t take the length of the membrane and unlike the Zonulas, does not surround the cell. It is a disk-shaped structure matched with an identical structure at the surface of the adjacent cell made up of 12 proteins (circular attachment plaques or spots). The membrane in this region are straight and usually further apart (30nm). It’s adhesion function is further empowered by groups of intermediate keratin filaments which provides firm adhesion.
5. Hemidesmosome literally means half a desmosome. It is also a Macula or plaque or spot of adhesion but between the basal surface of cells and their basal lamina (layer made of connective tissue usually present under cells in tissue). So in effect, the Maculais only on one side.

Intercellular Junctions.
From a functional point of view, therefore, junctions between cells can be classified as adhering junctions (zonula adherens, desmosome and hemidesmosome), impermeable junctions (zonula occludens) and communicating junctions(gap junctions).
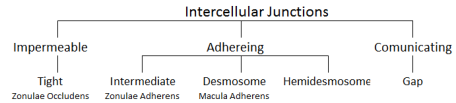
Intercellular Junctions Summary Classification.
Additionally, as mentioned earlier, intergrins are receptor proteins that both bind to and respond to the ECM in different ways such as wound healing, cell differentiation (chapter 10), and apoptosis (chapter 11).