The basic energy source of living things comes from sunlight, but animal cells cannot use sunlight energy directly, unless it is transformed into chemical energy. Chloroplasts (plant cell and algae organelle) change sunlight energy into chemical energy stored in organic substances such as carbohydrates. And animals and other cells do not have chloroplasts rather, get energy from breaking down those organic substances.
Mitochondria in animal cells are responsible for transforming the chemical energy into adenosine triphosphate (ATP) which cells can use directly.
What we should deduce from this is that both mitochondria and chloroplasts are energy producing organelles in cells. This is why they are generally called power houses.
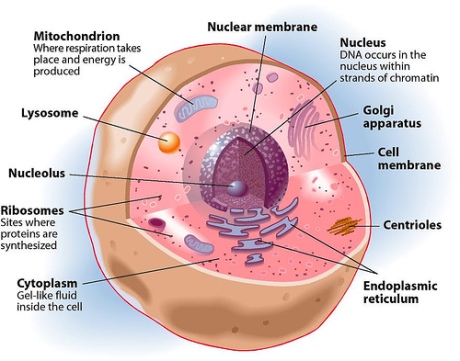
To understand how mitochondria functions to synthesize ATP, we have to study it’s sophisticated structure first.
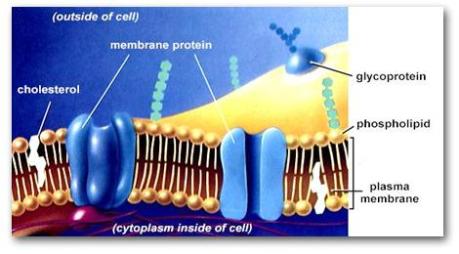
Mitochondria (singular mitochondrion) have two highly specialized membranes and an intermembrane space, and a large internal space called the matrix. The outer-membrane has many molecules of transport proteins which form gateways also called channel proteins, through to the inner-membrane. These gateways appear to be porous (like a sieve) and permeable to molecules of 5000 dalton (1 dalton = mass of 1 hydrogen atom).
The inner-membrane, however, is less permeable and contains integral proteins (integrin) that are selective to the molecules and compounds which can enter the mitochondrial matrix.
Because the outer-membrane is highly permeable and the inner-membrane very selective, the intermembrane space is similar to that of the cytosol, however, the mitochondrial matrix contents are highly specialized.
Most of the proteins embedded in the inner-membrane are components of the energy producing processes and therefore are highly convoluted (twisted in on itself to provide a larger surface area by covering very little space). These enfolding are known as cristae.
Once again, because the cristae are part of the inner-membrane and contain the proteins necessary for ATP synthesis, depending on the energy needed for the cell, the cristae may increase or decrease. For example, cardiac muscle cells have three times more cristae in their mitochondria than liver cells, because of greater demand for ATP.
In cells, mitochondria can have many different shapes (polymorphism) and are present in different quantities (variable), and can move around (mobility) and can produce ATP depending on the energy required in the cell (adaptability). In skeletal muscles, for example, the number of mitochondria can increase 5 to 10 times, if the muscle is required to move so much, as in regular exercise.
Biochemically, many chemical components of mitochondria are:
- Proteins which can be classified into two types:
- Soluble proteins; which are manly enzymes and peripheral proteins (Chapter 3).
- Insoluble proteins; which are integral proteins and cytoskeleton proteins (more in chapter 6).
2. Lipids, largely phospholipids.
However, one thing to note is that half of the outer-membrane is made of protein and the other half is made of lipid, while in the inner-membrane 80% is protein and the rest 20% is composed of lipid.